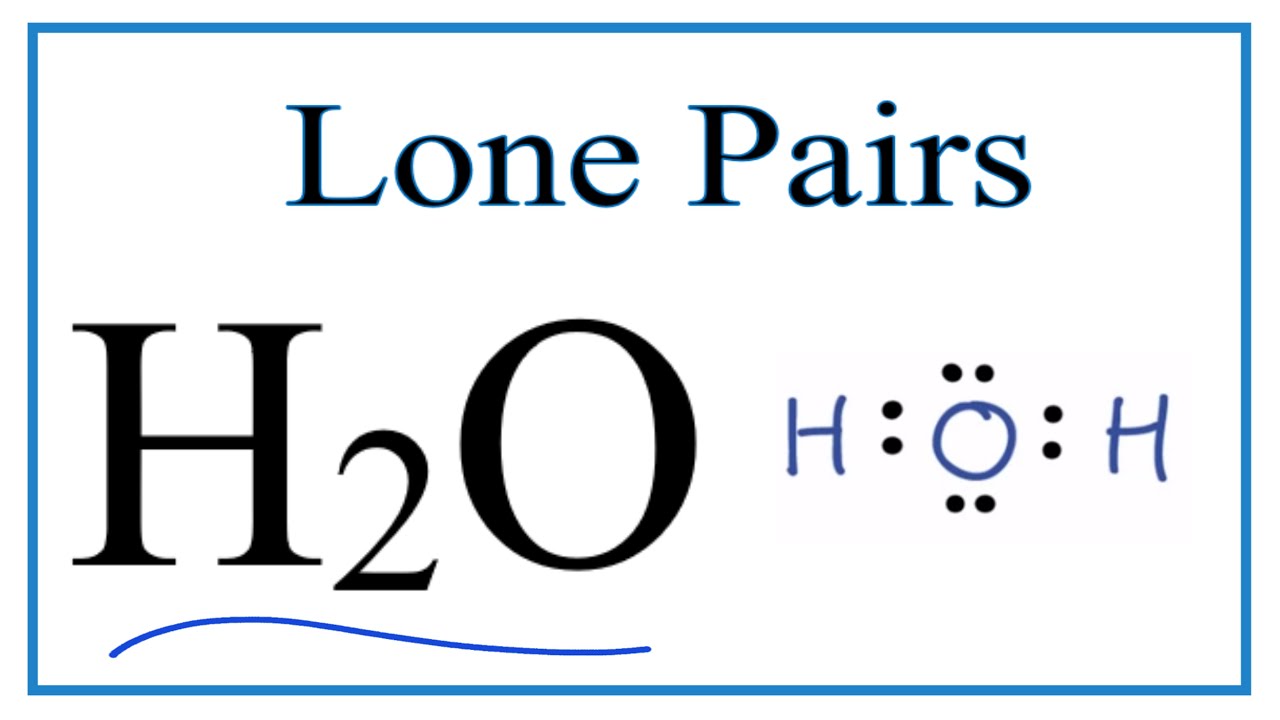Lone Pairs And Bonding Pairs
Solved lone pairs bonding pairs and in the lewis structure Lone pairs bonding bond slidesharetrick electron Pair lone bond between difference bonding electron sigma electrons unpaired valence orbital double single pi non comparison definition covalent examples
PPT - Covalent Bonding PowerPoint Presentation, free download - ID:5648526
Number of lone pairs and bonding pairs for h2o (water) Lone pairs molecular pi orbitals bonding which into go part second chemistry Pairs lone co2 lewis bonding structure band transcribed text show there
Lone shared pair electrons bonding electron bond pairs chemistry molecule unshared valence atoms hydronium bonded ion structure please show distributed
9.7: the shapes of moleculesLone bonding slidesharetrick easiest Lone bond covalent bondingBonding pairs and lone pairs.
Go to the ib physics pageOrganic chemistry Solved 1. identify the number of bonding pairs and loneValence shell electron pair repulsion theory.

Electron pair shapes theory vsepr molecules valence shell repulsion chemistry molecular bond linear shape geometry chart lone bonding geometries chem
Bonding bond lone ppt powerpoint presentation pairs pair slideservePairs lone bond pair identify becl2 atom chlorine vsepr theory molecules do description central chemistry Lone chemistry pair lewis pairs geometry molecular electron axe bonds atoms atom central geometries only method number diagram arrangement introductionWhat is lone pair and bond pair.
Bonding lone pairs electrons versus bondGeometry chemistry bond molecular lone pairs molecules bonding vsepr bent theory shapes electron model models angle shape molecule general pair 7.4: molecules with lone pairsLone pairs molecules bonding shapes chemistry pair molecule tetrahedral electron four arrangement libretexts which covalent.

Solved lone bonding identify problem
Pairs lone bonding slidesharetrick eleVsepr theory Bonding pairs and lone pairsBonding covalent pairs bond lone.
Bonding lone pairsWhat is a lone pair in a lewis diagram Lone h2o bondingBonding pairs and lone pairs.

Bonding lone pair shape lewis bf4 four charge pairs tetrahedral pyramidal centers there if answer hybridisation test hedberg numericable perso
Difference between bond pair and lone pair .
.